A new miocene–pliocene ichnotaxon for vermetid anchoring bioerosion structures
- 1Departamento de Paleontología, Instituto de Ciencias Geológicas, Facultad de Ciencias, Universidad de la República, PEDECIBA Geociencias, SNI-ANII, Montevideo, Uruguay
- 2Área de Paleontología, Departamento de Biología Animal, Edafología y Geología, Facultad de Ciencias, Universidad de La Laguna, Santa Cruz de Tenerife, Spain
- 3Museo de Naturaleza y Arqueología (MUNA), Organismo Autónomo de Museos y Centros, Santa Cruz de Tenerife, Spain
- 4Universidad Nacional de Río Negro, Instituto de Investigación Paleobiología y Geología (IIPG), Viedma, Río Negro, Argentina
- 5Consejo Nacional de Investigaciones Científicas y Técnicas, Instituto de Investigación Paleobiología y Geología, General Roca, Río Negro, Argentina
- 6Department of Earth Sciences, Faculty of Experimental Sciences, Campus de El Carmen, University of Huelva, Huelva, Spain
- 7Department of Apply Geosciences, CCTH - Science and Technology Research Centre, University of Huelva, Huelva, Spain
- 8Department of Geology, Faculty of Geology, University of Oviedo, Oviedo, Spain
A revision of Renichnus arcuatus Mayoral, 1987, the vermetid attachment etching trace fossil (fixichnia), is presented here with an emended diagnosis. Renichnus arcuatus should be used only for nested reniform depressions arranged in linear series or solitary ones. A new ichnotaxon, Santichnus mayorali ichnogen. et ichnosp. nov., is described to name a bioerosion structure that previous authors included under R. arcuatus. The new trace fossil comes from the Miocene–Pliocene deposits from Fuerteventura and Lanzarote, Canary Islands, and is characterized as a shallow canal, semicircular in cross-section that occurs on the surface of hard substrates. Santichnus mayorali follows a logarithmic spiral path that may depart in its outer whorl in a somewhat straight shaft that becomes recurved back toward the spiral. From an actualistic point of view, this new ichnotaxon is interpreted as the anchorage bioerosion structure of vermetid gastropods. Given the close relationship between the two ichnotaxa (Renichnus and Santichnus) that share vermetid gastropods as their tracemakers, it is proposed that they should be considered as compound trace fossils when they occur interconnected.
Introduction
Organisms attached to the substrate can be found in continental or marine environments but are more frequent in the last one. Living in the sea poses some problems as strong waves or currents may transport the organism to unfavorable areas or render it susceptible to predation. Anchoring to the substrate is a mode of life that tends to mitigate those problems. The attached organisms have developed various strategies to anchor themselves to the substrate. Some are anchored temporarily, employing grasping appendages or muscular suckers; others attach to the substrate permanently by cementing to it. For a detailed review of attachment strategies to hard substrates, see Bromley and Heinberg (2006). The action of anchoring to a hard substrate involves some etching very frequently, which in turn results in bioerosion. Bioerosion structures encompass five ethological categories. The great majority belong to the domichnia or domicile borings, made for protection, which commonly penetrate relatively deep into the substrate and, in most cases, cover the whole organism. Less common are those included in the pascichnia, which are produced by herbivorous or omnivorous grazing animals, producing surficial scratching marks on different substrates. Praedichnia includes predatory trace fossils that encompass borings, scratching, biting, breaking, and smashing marks emplaced on skeletal material. The equilibrichnia was erected for those traces that keep pace with an accreting surface (de Gibert et al., 2004). The fifth category, the fixichnia, was established by de Gibert et al. (2004) to group those bioerosion structures resulting from attachment to a hard substrate. The bodies of producers of anchoring structures are not completely covered by the substrate since they only etch the surface. Hence, the common architectural characteristic of anchoring structures is that they are shallow surficial structures. Examples of this kind of trace fossils are the ichnogenera Camarichnus (Santos and Mayoral, 2006), Canalichnus (Santos and Mayoral, 2006), Centrichnus (Bromley and Martinell, 1991), Finichnus (Taylor et al., 2013), Podichnus (Bromley and Surlyk, 1973), Renichnus (Mayoral, 1987), Spirolites (Uchman et al., 2018), Stellichnus (Mayoral, 1987), and Sulcichnus (Martinell and Domènech, 2009). In this article, we review the use of the name Renichnus arcuatus (Mayoral, 1987), a monoichnospecific ichnogenus in the ichnologic literature. This name has been extended by other authors to bioerosion structures beyond the original diagnosis of the ichnogenus. A product of this revision is the description of a new bioerosion structure from the Miocene and Pliocene of the Canary Islands, attributed to vermetid gastropods.
Study area, geological setting, age, and paleoenvironments
The Canary Islands are an intraplate volcanic archipelago located northwest of the African continent formed along the Neogene and Quaternary periods (Figure 1A). The easternmost islands, Lanzarote and Fuerteventura (Coello et al., 1992; Gutiérrez et al., 2006), closest to the African coast, are also the oldest (23–15 Ma).
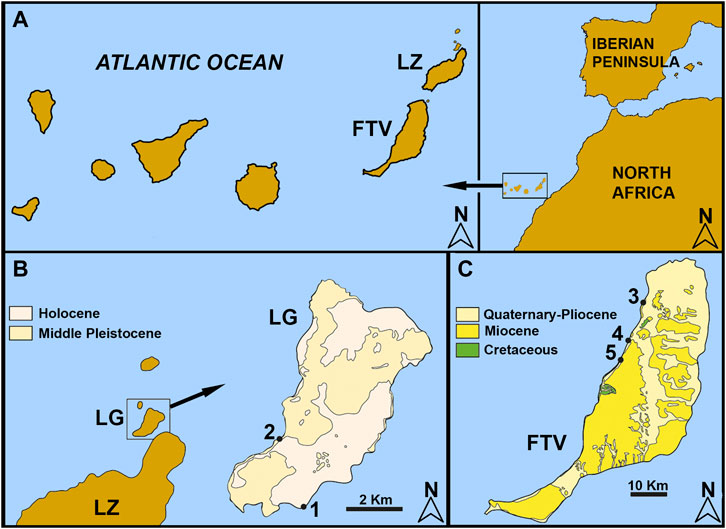
FIGURE 1. Geographic location of the studied material. (A) the Canary Islands and their global location. (B) location and geology of La Graciosa Island with fossiliferous sites. (C) geological map of Fuerteventura Island and paleontological site location. 1, Baja del Corral; 2, Playa del Salado; 3, Aljibe de la Cueva; 4, Barranco León; 5, Playa del Valle.
The Island of La Graciosa, together with the islets of Alegranza, Montaña Clara, Roque del Este, and Roque del Oeste, makes up the Chinijo Archipelago off the north coast of the island of Lanzarote. This archipelago is composed of a volcanic sequence of cinder cones and hydromagmatic centers on a flat platform. The sequence goes from the lower Pleistocene and upper Pleistocene–Holocene (De la Nuez et al., 1997a; Castillo et al., 2002; Ortiz et al., 2006). La Graciosa is the largest island, 27 km2 in area, where the oldest volcanic rocks appear under more than 1 m of fossil beach (Ortiz et al., 2006). The sites studied here are located in the Pleistocene sediments of the southeast coast (Playa del Salado), and the Holocene sediments of the southwest coast (Baja del Corral; Figure 1B).
The stratigraphic sequence of the deposits studied on La Graciosa begins with a paleosol developed on a lava flow. This is followed by a level of sand and a level of conglomerates with marine fossil remains, after which a series of intercalated sand and paleosol can be observed (De la Nuez et al., 1997b; Yanes et al., 2004; Ortiz et al., 2006).
Fuerteventura has an older history that begins with Mesozoic marine sediments from Ajuy (Gutiérrez et al., 2006). According to Martin-González et al. (2001) the transgressive deposits are found atop basalts dated between 17 and 11.8 Ma. The basaltic flows that cover these deposits are Pliocene, dated between 4.83 and 1.9 Ma. The sites studied here extend along the west coast of the island, from Aljibe de la Cueva beach in the northwest to Playa del Valle in the southwest (Figure 1C).
The stratigraphic sequence of the deposits studied on the island of Fuerteventura begins with a marine conglomeratic level deposited on the Miocene volcanic materials, which correspond to the first subaerial volcanic phase (Martin-González et al., 2018). The Aljibe de la Cueva site (Figure 1C) is of great lateral extension. The conglomerate strata alternate with decreasing grain sequences with gravels that include horizons of rounded pebbles and rhodoliths, breccias, and a paleosol immediately below a Pliocene capping lava flow (Martín-González et al., 2018). The fossils here studied are in the conglomerate and rhodolith beds. In Barranco León site (Figure 1C), the conglomeratic level is located above the fossil marine platform. South of this ravine, the marine deposits are not covered by Pliocene basaltic flows but by sandy deposits that are interpreted as fossil eolianites, which are interspersed with conglomerates and gravel that are interpreted as levels formed in wet alluvial fans (Meco et al., 2015). At this site, the fossils were collected from the conglomerate and in the sand levels. Last, at Playa del Valle (Figure 1C), the conglomerate level is supported by pillow lavas and breccias of pillow fragments traversed by deep-seated dikes of the Submarine Volcanic Group (Gutiérrez et al., 2006). In the middle part of the section is a bank of medium sand corresponding to beach horizons, on which lies a very cemented bioclastic level composed of a large number of small mollusk shells and molds and a low proportion of volcanic rocks. The sequence continues with breccia–conglomerate packages. At Aljibe de la Cueva, the fossils were found at the conglomerate level.
Materials and methods
The studied material is housed at the Paleontological Collection of the Universidad de La Laguna, San Cristobal de La Laguna, Tenerife Island, Canary Islands, Spain, under the acronyms ULL PA, IG, and FPA. The fossils analyzed were found on mollusk shells in the collection at the Paleontology Area of the University of La Laguna, Tenerife, and also in the field at Playa del Valle, Barranco León, and Aljibe de la Cueva sites. For preparation, the material was cleaned mechanically with brushes and washed under tap water when the fossils were resistant to this process. Those materials that underwent SEM sessions using a JEOL 5900 Low Vacuum electronic microscope were coated in order to gain maximum image quality. Schematic drawings of fossil silhouettes to represent overall shapes were made over the photographs using an image editor.
Systematic ichnology
A revision of the literature was made to check the use of the name Renichnus by previous authors. It was evident that this name has been applied to fossil bioerosion structures of notoriously different morphologies. On one side, the series of nested reniform depressions was as in the original description of ichnogenus Renichnus (Mayoral 1987) but also some shallow channels that follow a spiral path. We propose an emended diagnosis and use this name only for the nested reniform depressions. These spiral shallow trace fossils are described below as a new ichnogenus.
Repository. All the specimens revised, analyzed, described, and illustrated in the following section are housed in the palaeontologic collections of the Paleontology Area of the University of La Laguna (Tenerife, Spain) with assigned registration numbers FPA 1–10, IG 9, 19, 20, 34, and 136 and ULL PA 226, 231, 307, 465–469, 471–473, 481, 486, 501–503, 506, and 520.
Ichnofamily Renichnidae (Wisshak et al., 2019).
Type Ichnogenus Renichnus (Mayoral, 1987).
Ichnogenus Renichnus (Mayoral, 1987)
Vermetus intortus etchings (Radwański, 1969, p. 44, Plate 8, Figures 5, 6. Miocene, southern Poland).
Petaloconchus intortus etchings (Radwański, 1977, p. 247, Plate 9 a2, b2, c2, d2, e, f2, and Plate 10 a, b, c, and e (one specimen near upper right corner). Miocene, southern Poland).
Renichnus arcuatus (Mayoral, 1987, p. 56, Plate II-13. Lower Pliocene, SW Spain).
Etching by Petaloconchus (Macrophragma) cereus (Savazzi, 1996, p. 160, Text-Figure 2F. Recent, Bantayan island, The Philippines).
Renichnus arcuatus (Taddei Ruggiero, 1999, p. 171, Figures 1O,P. Upper Pliocene, Apulia, Italy).
Renichnus arcuatus (Jagt, 2003, p.177, Plate 2, Figure 3. Maastrichtian, Bemelen, Netherlands).
Renichnus arcuatus (Donovan, 2003, p.137, Figures 1–3. Pleistocene, north-central Jamaica).
Renichnus arcuatus (Santos and Mayoral, 2006, p. 728, Plate 3–8. Lower Pliocene, Southern Spain).
Renichnus ichnosp. (Matamales-Andreu et al., 2007, p. 23, Plate 1-b. Upper Pleistocene, Mallorca, Spain).
non or dubious Renichnus arcuatus (El-Hedeny, 2007), p. 12, Plate 3 Figures 7, 9. Cenomanian-Campanian, Sinai, Egypt).
Renichnus arcuatus (Taddei Ruggiero and Bitner, 2008, p. 370, Figure 4C. Upper Pliocene, Moncalvo, Italy).
non Renichnus ichnosp. (Hoșgör and Okan, 2010, p. 51, Plate 1 Figure 2. Early-middle Miocene, SE Turkey).
Renichnus arcuatus (Donovan et al., 2011, p. 104, Figures 5A, 7. Maastrichtian, Belgium, and the Netherlands).
non Renichnus arcuatus (Wisshak et al., 2011, p. 507, Figure 9K. Recent, Azores Islands, Portugal).
non Renichnus ichnosp. (Uchman et al., 2017, p. 46, Figure 8f-g. Lower Oligocene, northern Italy).
Other references to this ichnotaxon with no figured material:
Renichnus arcuatus (Bromley and Asgaard, 1993, Pliocene, Island of Rhodes, Greece).
Renichnus (Dávid et al., 2008, Early Miocene, Tardona Hills, Hungary).
Type ichnospecies Renichnus arcuatus (Mayoral, 1987) by monotypy.
Renichnus arcuatus (Mayoral, 1987) Figures 2A,D; Figures 3G,H.
Original description: “Set of more or less shallow scars, in an aligned form or a sinuous to spiral trajectory (Figures 2A–D, 3G–H). They may occur isolated or in groups. The scars are usually smaller at one end (and much more regular, with an elongated, ovoid outline) and larger at the opposite end. In this case, the impressions are distinctly reniform, with one side highly arched.”
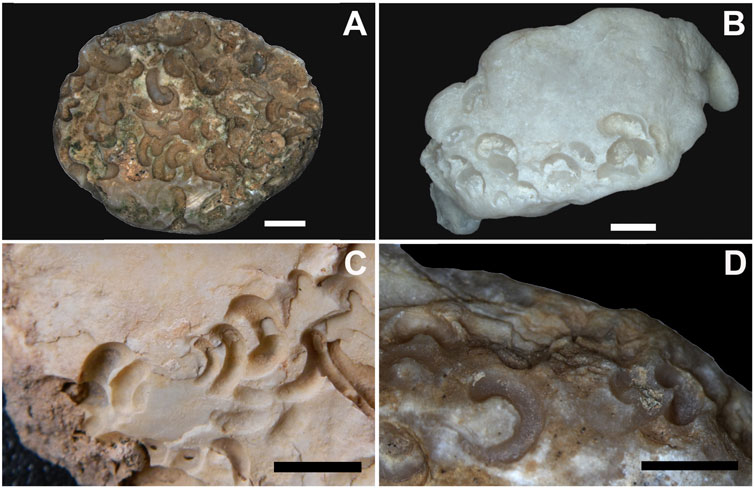
FIGURE 2. Examples of Renichnus arcuatus Mayoral, 1987 from three of the studied localities. (A) ULL PA 520 (Barranco León). (B) ULL PA 473 (Playa del Valle). (C) ULL PA 501 and (D) ULL PA 502 (Playa Aljibe de la Cueva). Scale bars: 5 mm.
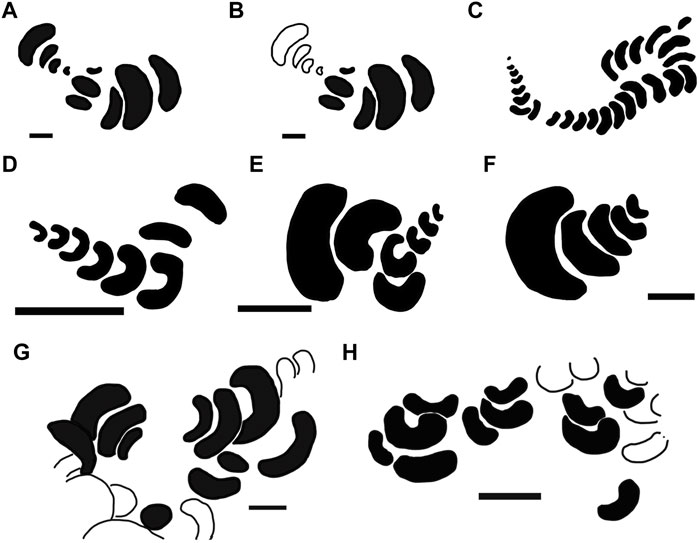
FIGURE 3. Schematic drawings of variants of Renichnus arcuatus (Mayoral, 1987), based on different occurrences from the literature (A–F) and this study (G–H). (A,B): type material of R. arcuatus (Mayoral, 1987) (Pliocene, Spain). (A) whole sample with holotype and paratype. (B) holotype highlighted in black. (C) Miocene of Poland (Radwański, 1969; 1977). (D) Maastrichtian of the Netherlands (Jagt, 2003). (E) Maastrichtian of Belgium (Jagt, et al., 2009). (F) Maastrichtian of Belgium (Donovan et al., 2011). (G) Playa del Aljibe, Fuerteventura, Spain, ULL PA 501. (H) Playa del Valle, Fuerteventura, Spain, ULL PA 473. White traces in (G,H) correspond to incomplete scars. Scales: (A,B): 1 mm; (C) approximately 55–60 mm maximum in length; (D–F,H) 5 mm; (G) 2 mm.
Emended diagnosis: Bioerosion structures that develop on the surface of calcareous hard substrates as nested reniform or crescentic depressions arranged in straight, sinuous, or strongly bent series or rows. Solitary depressions may also occur. The size of the depressions can be constant or vary in a gradual manner, reducing in size toward the concave-pointing end of the nested series.
Remarks: As originally defined, ichnogenus Renichnus (Mayoral, 1987) and its only known ichnospecies, Renichnus arcuatus (Mayoral, 1987), should be used only to refer to a series of reniform depressions. These traces are generated when a high trochospiral shell (Figure 4A) attaches to the substrate with the coiling axis parallel or tangential to the attachment surface. The result is a series of nested reniform or crescentic depressions arranged in rows. Those depressions represent the area where each whorl makes contact with the substrate. The materials that Mayoral (1987) used in this trace fossil’s original description and illustration are the holotype (BO2/1/2) and two paratypes (BO1/2/08; LU2/2/19). Since Renichnus is a trace fossil and despite representing the attachment of a trochospiral shell but not the shell itself (although it often maintains part of it attached), we recommend eliminating the term “spiral” from its diagnosis. As mentioned above, there are neither true spirals nor true whorls in the trace fossil morphology since the depressions are reniform. The original wording of the diagnosis, including the term spiral, probably led other authors to include disparate materials in this ichnogenus that we propose to be treated as a new ichnotaxon since it represents a different behavior when the animal anchors itself to the substrate in a different way, as will be explained below. The producers of these series of nested reniform depressions are vermetid gastropods such as Petaloconchus intortus (fossil), P. sculpturatus (fossil), P. glomeratus, and P. laurae, which have high trochospiral shells (Radwański, 1969, Radwański, 1977; Mayoral, 1987). Vermetids are known for their shell coiling plasticity, so some species may show individuals with trochospiral shells, others with planispiral shells, and even individuals with shells that bear the two patterns. Thus, some intergradation between Renichnus and the new ichnogenus described below may be expected.
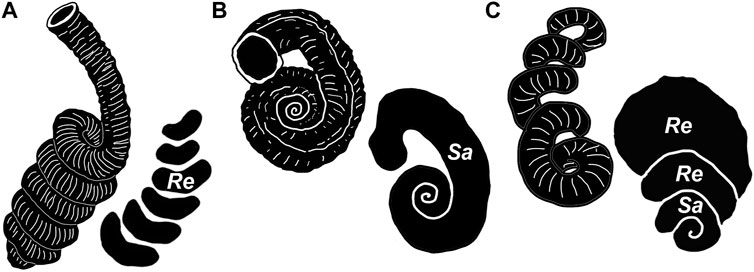
FIGURE 4. Idealized vermetid shell types responsible for the ichnogenera treated here. (A) Petaloconchus-like high trochospiral coiling responsible for Renichnus arcuatus (Mayoral, 1987). (B) Serpulorbis-like planispiral coiling responsible for Santichnus mayorali. (C) irregularly coiled vermetid shell that combines a tight spiral and loose whorls (based on ULL PA 458, see Panel 8A), responsible for compound forms of Renichnus–Santichnus. (Re: Renichnus; Sa: Santichnus) Not to scale.
Santichnus, Verde, Castillo, Martín-González, and Cruzado-Caballero. Ichnogen. nov.
Etymology: Dedicated to Ana Santos, a Portuguese ichnologist, and appreciated colleague, who has made numerous relevant contributions in the field of bioerosion.
Diagnosis: Bioerosion structure that develops on the surface of hard calcareous substrates as a canal semicircular in cross-section that follows a logarithmic spiral path up to three whorls, in which the width of the canal varies gradually following the logarithmic spiral proportions.
Remarks: Santichnus can be easily distinguished from Spirolites Uchman et al. (2018), another anchoring spiral trace fossil produced by vermetid gastropods. Santichnus has a distinguishable discrete canal along its spiral path and lacks the perpendicular annulations in contrast to Spirolites Uchman.
Type ichnospecies Santichnus mayorali ichnogen. nov., ichnosp. nov.
Santichnus mayorali Verde, Castillo, Martín-González and Cruzado-Caballero. Ichnosp. nov.
Figures 5A–G; Figures 6A–H. Figure 7A-L.
Other occurrences: Here, we list additional occurrences of Santichnus mayorali from the literature other than the studied localities listed above.
Pomatoceros etchings (Radwański, 1969, pp. 43–44, Plate 8, Figures 3, 4. Miocene, Southern Poland).
Serpulid etchings (Radwański, 1977, p. 247, Plate 10 d and e [several specimens associated with one Renichnus arcuatus near the upper right corner]).
Conchas con marcas superficiales que consisten en pequeñas galerías espirales de una única vuelta de espira (shells with shallow marks with the shape of small spiral galleries with only one whorl) (Martín-González et al., 2001, p. 51).
Embedment of a coiled serpulid worm (Donovan, 2003, p.138, Figure 2 [left]. Pleistocene, Jamaica).
Dubious material Renichnus arcuatus (El-Hedeny, 2007, p. 12, Plate 3, Figures 7, 9. Cenomanian-Campanian, Sinai, Egypt).
Renichnus ichnosp. (Hoșgör and Okan, 2010, p. 51, Plate 1 Figure 2. Early-middle Miocene, SE Turkey).
Renichnus arcuatus (Wisshak et al., 2011, p. 507, Figure 9 k. Recent, Azores Islands, Portugal).
Renichnus ichnosp. (Uchman et al., 2017, p. 46, Figure 8 f-g. Lower Oligocene, northern Italy).
Holotype: Sample FPA-7, one fragment of patellid with the holotype FPA-7 K and several additional specimens on its inner side.
Paratype: ULL PA 469, one fragment of ostreid with the paratype and other specimens along with Renichnus.
Etymology: In honor of the esteemed colleague Eduardo Mayoral, a Spanish ichnologist who developed a pioneer research activity on bioerosion structures and is also the author of ichnogenus Renichnus, which is revised here.
Type material locality: The holotype FPA-7 K comes from the base of the Barranco León site and the paratype ULL PA 469 comes from the Playa del Valle site, both on the northwest coast of Fuerteventura Island.
Stratigraphic position and age of type material: The holotype FPA-7 K comes from an unnamed cemented sand level of Miocene–Pliocene age and paratype ULL PA 469 comes from an unnamed bioconglomerate (rhodolith level) of Miocene–Pliocene age.
Diagnosis: Bioerosion structure developed on the surface of hard substrates as a canal semicircular in cross-section following a spiral path of up three whorls, in which the width of the canal varies in a gradual manner following logarithmic spiral proportions. The spiral lies with its coiling axis perpendicular to the substrate surface and the last whorl may depart from the spiral coil in a straight shaft or a recurved one that returns back toward the spiral. A nonbioeroded subtriangular to crescentically arched area may be present between the recurved shaft and the spiral. Reniform depressions similar to those of Renichnus can be present around the last whorls in some specimens. The canal may show fine striae perpendicular to the central axis of the canal.
Description: The holotype, FPA 7 K (Figures 5A–C), occurs on the inner side of a patellid fragment (Figures 5A,B). It is a shallow bioerosion structure consisting of a channel semicircular in cross-section following a logarithmic spiral path with 3.5 whorls. The spiral lies with its coiling axis perpendicular to the substrate surface, ending in a departing shaft and recurved hook that returns toward the spiral reminiscent of some scaphitid ammonoids. This recurved hook delimits a roughly triangular nonbioeroded area between it and the last whorl of the spiral (Figures 5C, 7A). The depth of the channel varies in proportion to its width along its path. The width of the channel varies gradually along the spiral and is 1.1 mm at maximum. The spiral is 3.2 mm in diameter in its outer limits, sinistral as observed from outside the substratum.
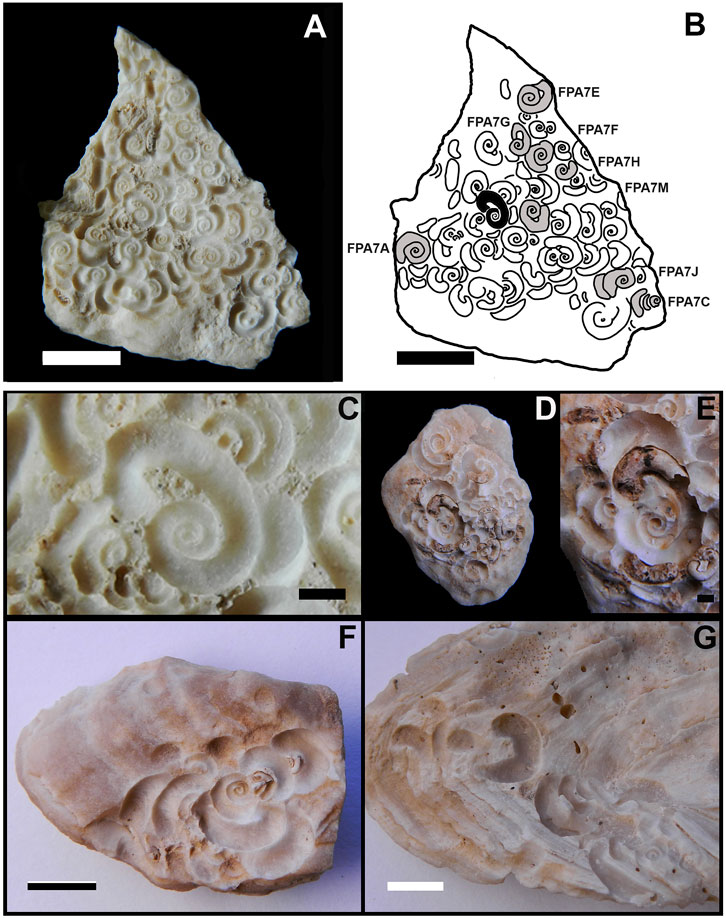
FIGURE 5. Santichnus mayorali ichnogen. nov., ichnosp. nov. (A) whole sample bearing holotype (FPA 7 K). (B) holotype location is highlighted in black on the sample and other specimen locations on the same surface. (C) detail of holotype. (D,E): paratype (ULL PA 469). (D) whole sample with paratype and other specimens. (E) detail of paratype. (F) three clearly compound specimens of Renichnus–Santichnus (ULL PA 472). (G) Specimens of Renichnus and Santichnus (lower right corner), occurring closely but not clearly in a compound association (ULL PA 467). Scale bars: (A,B,D) 5 mm and (C,E) 1 mm.
The paratype, ULL PA 469 (Figures 5D,E, 7B), occurs on an ostreid fragment (Figure 5D). It consists of a shallow bioerosion structure that is a channel semicircular in cross-section that follows a spiral path with 3.5 whorls plus the departing shaft and recurved hook, lying with its coiling axis perpendicular to the substrate surface (Figure 5E). The maximum width of the channel is 1.5 mm, and the spiral is 5.4 mm in maximum external diameter, sinistral as observed from outside the substratum. It has the retroverted terminal hook that delimits a nonbioeroded, subtriangular area between it and the spiral and has reniform scars, one running parallel to the recurved hook and two on the opposite side.
Other analyzed specimens show that the last whorl of the spiral may depart at different angles and that the nonbioeroded area can vary in shape from subtriangular to arched or lunate (Figures 6A,C,E,F,H; Figures 7A–C,E–L). When the Santichnus mayorali spirals have attached the reniform scars of Renichnus arcuatus, the arrangement of these elements can vary. These reniform scars may be located only parallel to the retroverted hook (Figures 6A,B,H, 7C–E) or in this same position and also on the opposite side, like the paratype ULL PA 469 (Figures 5D,E). In some specimens, the spiral structure lies in a nested arrangement inside the reniform Renichnus scars, forming a compound specimen as in the paratype or in others as in ULL PA 472 (Figure 5F). When a spiral is preserved disconnected from reniform scars, it may be difficult to discern whether they belong to one specimen or more than one as in ULL PA 467 (Figure 5G).
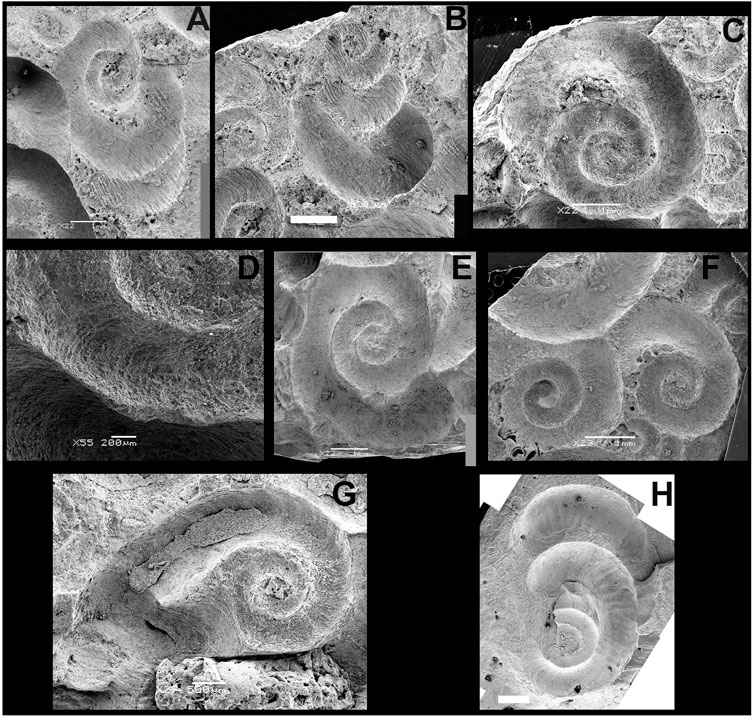
FIGURE 6. SEM images of Santichnus mayorali ichnogen. nov., ichnosp. nov. showing different morphological variants. (A) FPA 7 J and (B) FPA 7 C with xenoglyph of the substratum mollusk shell. (C) FPA 7 E. (D) Detail of FPA 7 E showing fine grooves and ridges. (E) FPA 7 A. (F) FPA 7 H on the right. (G) FPA 7 G with fine grooves and ridges. (H) FPA 1 showing fine grooves and ridges, associated with a reniform scar in its last whorl (upper part of the specimen). Scale bars: (A–C, E–F,H), 1 mm; (D) 200 microns, (G) 500 microns.
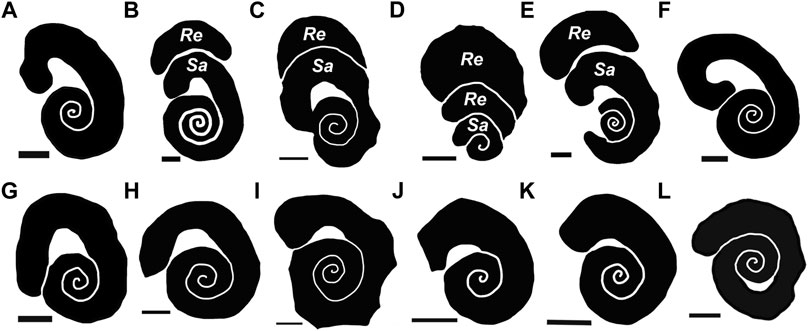
FIGURE 7. Schematic drawings of Santichnus mayorali showing variation based on the studied material. Specimens with a spiral with shaft and recurved hook. (A) holotype (FPA 7K). (B) paratype (ULL PA 469). (C) (FPA 7J). (D) (FPA 7C) (E) (FPA 1). (F) (FG 19). (G) (FPA 7M). (H) (FPA 7E). (I) (FPA 7A). (J) (FPA 7H). (K) (FPA 7D). (L) (FPA 7F). Re: R. arcuatus; Sa: S. mayorali. Scale bars: 1 mm.
Fine grooves perpendicular to the axis of the channel may be found in some specimens (Figures 6C,D,G,H) as in specimens FPA-7 E, FPA-7 H, and FPA-1. Xenoglyphs copying the texture of the substrate can be seen on some specimens (Figures 6A,B).
Remarks: Santichnus mayorali can be distinguished from other bioerosion ichnotaxa with spiral elements in their architecture. It differs from the similar trace fossil Spirolites radwanskii (Uchman et al., 2018), another fixichnion, because of its evolute spiral path in contrast to that of Spirolites, which is involute. Santichnus mayorali shows a discrete channel, unlike Spirolites radwanskii. Besides this, Spirolites have first- and second-order annuli that result in an annulated surface, lacking in the material described here. Santichnus mayorali may also show a path departure from the coiling, ending in a hook, absent in Spirolites. In addition, Spirolites only reach up to 1.3 whorls; whereas Santichnus can have up to 3.5 whorls. The spiral of Santichnus does not crosscut its previous path as in Spirolites.
Finichnus tortus (Rosso, 2008), another fixichnion, with spiral architecture can also be easily distinguished from Santichnus. Finichnus tortus is composed of a series of shallow, oval to pyriform depressions arranged in a spiral pattern, and these are absent in the new ichnotaxon.
Other bioerosion ichnotaxa with spiral patterns such as Spirichnus spiralis (Fürsich et al., 1994) and Maeandropolydora barocca (Bromley and D’Alessandro, 1987) should, in principle, not be confused with Santichnus. These ichnotaxa do not represent superficial bioerosion structures but are true borings that deeply penetrate the substrate, which also implies a different type of behavior, that of creating shelter, belonging to the ethological category domichnia.
Santichnus and its only known ichnospecies, S. mayorali, should be used only for shallow bioerosion structures in which a canal semicircular in cross-section follows a spiral path. These traces are generated when a planispiral shell contacts the substrate with the spiral axis perpendicular to the attachment surface (e.g., Figure 4B).
Actualistic data on producers and the architectural plan of Santichnus mayorali that affects only the surface of the substrate suggests that this ichnotaxon can be classified as an anchoring bioerosion structure, belonging to the ethological category fixichnia (de Gibert et al., 2004).
Renichnus–Santichnus compound trace fossils and tracemakers
Vermetid gastropods are known for their ability to colonize hard substrates. When anchoring to hard substrates, they etch the surface removing material and cement directly to it (Savazzi, 1996, 1999; Bromley and Heinberg, 2006). Bromley and Asgaard (1993) mentioned that Renichnus is produced by sessile mollusks etching the substrate surface chemically. Savazzi (1996) mentioned that the vermetid bioerosion mechanism is unknown, but their weak radula and thin chitinous operculum suggest that mechanical abrasion is not the main cause of bioerosion. The reabsorption of the shell’s last whorl surface prior to the mineralization of the subsequent one would be the evolutionary origin of the bioerosion of substrates in vermetid gastropods (Savazzi, 1999). From the Upper Cretaceous (Maastrichtian) to the present day, there have been numerous occurrences of kidney-shaped or crescentic traces that have been assigned to Renichnus, which usually occur in isolation or in small groups. Bromley (2004) suggested using the name Renichnus (Mayoral, 1987) both for, a series of reniform depressions and for spiral canals made by vermetids since intermediate forms are found. However, not all occurrences of reniform scars and spiral canals show this intergradation. In some cases, Renichnus is directly related to the new trace fossil described here as Santichnus (Figures 5D–F, 6A,H), where the spiral is nested inside the reniform scars. This arrangement suggests that both the reniform scars (Renichnus) and the spirals (Santichnus) are the work of the same individual.
Radwański (1977) pointed out that the shell morphology of vermetids controls the shape of their etching scars on the substrate to which they anchor. It is on this premise, as well as the different behaviors vermetid gastropods display when they anchor to the substrate (Figure 4), that we base our proposal of separating two distinct morphologies of bioerosion structures, namely, Renichnus and Santichnus.
The overall spiral shape with the terminal hook is typical of vermetid anchoring etchings, as in several species of Thylacodes (T. arenarius and T. sulcatus) and Dendropoma (D. gregarium, D. cristatum, and D. petraeum). In the Canary Islands, an encrusted and bored specimen of Thais haemastoma (ULL PA 458), from Punta Negra, Tenerife Island (Figures 8A,B), shows specimens of vermetids that have etched reniform depressions and spirals in the same pattern as Santichnus mayorali’s path of a logarithmic spiral with a departure in the shape of a hook.
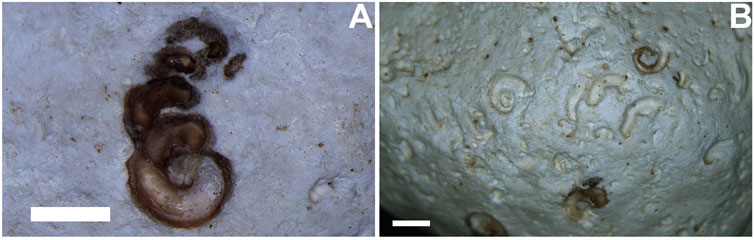
FIGURE 8. Vermetid gastropod associated with bioerosion on encrusted Thais haemastoma (ULL PA 458) from Punta Negra, (Tenerife Island). (A) vermetid gastropod with tight and loose coiling, responsible for compound forms of Renichnus–Santichnus (compare with Panel 4C). (B) the same specimen of Thais haemastoma with Renichnus and Santichnus and remains of vermetid gastropods. Scales: 2 mm.
In addition, Santichnus-like traces could correspond to vermetids that were partially or totally cemented to the substrate. In the first case, it may be in juvenile stages, which present planispiral coiling (Figure 4B), like Serpulorbis (s.s), which tends to remain attached to the substrate (Savazzi, 1996). By contrast, when the coiling relaxes and becomes more irregular as in adults, the resulting anchoring structures are Renichnus-like traces. This also occurs in Thylacodus, Trypsicha, and Petaloconchus (s.s.), which in their adult stages develop a stacking of successive whorls detached from the substrate, giving rise to regular to irregular conical shells (Figure 4A). In the second case, some vermetids may spend their entire lives planispirally attached, such as Serpulorbis (Cladopoda) (Keen, 1961) and Dendropoma sp. (Savazzi, 1996).
Petaloconchus (Macrophragma) coils similarly to Petaloconchus (s.s.). Still, its spire is laterally compressed and usually attaches to the substrate along one side rather than its apical whorls alone. This arrangement results in Renichnus-type traces, where Santichnus is not appreciable or preserved (Savazzi, 1996, Text-Figure 2F, p. 160).
Other case studies of fossil compound bioerosion structures are already known in the literature. Wisshak (2017) described microbioerosion structures (Dendrina × Filuroda) that were interpreted as produced by the same microorganism displaying different bioeroding behaviors.
Therefore, the Renichnus × Santichnus association as interconnected specimens would constitute a clear example of a compound trace fossil, where the organism, as it becomes an adult, may leave one type of trace or another depending on the growth pattern it develops (Figures 4C, 8A). This behavior change would occur successively where one ichnotaxon (Santichnus) passes intergradationally to another (Renichnus). If analyzed under the ichnogeny concept (Belaústegui et al., 2016; Belaústegui et al., 2020), these Renichnus × Santichnus compound specimens would constitute a record of an ichnogenetic sequence related to ontogeny. During the juvenile stages, the vermetids would generate Santichnus, whereas in more mature stages the gastropods would make Renichnus.
In this way and following the proposals of previous authors (Pickerill 1994; Pickerill and Narbonne, 1995; Buatois and Mángano, 2011), the resulting structure will be named in each case with the primary descriptor reflecting the dominant and diagnostic ichnotaxon, leaving the subsidiary name for its minor and integrated morphotypes. In turn, the names Renichnus and Santichnus should be used in solitary when the two morphotypes occur isolated.
Conclusion
The new trace fossil Santichnus mayorali ichnogen. nov. ichnosp. nov. is a vermetid anchoring etching bioerosion structure belonging to the ethological category fixichnia.
The name Renichnus arcuatus (Mayoral, 1987) has been used by different authors both as in the original description to identify a series of kidney-shaped depressions and grooves semicircular in a cross-section with a spiral path. Here, we have revised the synonymy of Renichnus arcuatus (Mayoral, 1987) and, based on the new ichnotaxon established in this article (Santichnus mayorali), proposed to treat them separately.
Renichnus arcuatus (Mayoral,1987) is made when a trochospiral shell lies with its coiling axis parallel or slightly inclined to the substrate surface. Santichnus mayorali is generated when a planispiral shell lies with its coiling axis perpendicular to the substrate surface.
These two morphologies (serially arranged nested kidney-shaped depressions and spiral canals) can be distinguished and can occur both interconnected or completely isolated in the fossil record. For this reason, Santichnus and Renichnus constitute a compound trace fossil when both occur interconnected, and following the established terminological guidelines for these cases, they should be named separately. Keeping the two names helps to distinguish different origins in anchoring behavior for each morphology, and that is especially useful when they are found isolated.
Vermetids show plasticity in shell morphology from planispiral to trochoid or contorted, and the separation of two ichnotaxa even in the situation of the same trace maker, is justified. Those trace fossils can occur interconnected by different coiling of one shell or, by contrast, by completely isolating without the intermediation of any taphonomical process, which is only through ethological and anatomical means.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material; further inquiries can be directed to the corresponding author.
Author contributions
MV, CC, EM-G, PC-C, EM, and AS studied the conception and design gathered. MV, CC, EM, and AS compiled the data acquisition. MV prepared ichnological data and figures. MV, CC, EM-G, PC-C, EM, and AS contributed to the discussion of results and shared the writing of the manuscript.
Funding
The Asociación Universitaria Iberoamericana de Postgrado (AUIP-España) and PEDECIBA Geociencias (Uruguay) partially funded MV visits to the Universidad de La Laguna (Tenerife Island) during 2018 and 2019, where this research was partially carried out. Project FCT-17-12775, FECYT. Ministry of Science, Research and Universities. Short-stay grant from the Vice-rectorate for Research at the University of La Laguna. EM and AS have been partially supported by the Research Project PID 2019-104625RB-100 of the Ministerio de Ciencia e Innovación of Spain, the Research Group RNM276 of the Junta de Andalucía (Spain), and the Science and Technology Research Centre, University of Huelva.
Acknowledgments
"We thank the handling editor SW, OV, and ML as referees and an anonymous reviewer who helped to improve our work. MV thanks SNI-ANII for the research support. MV, CC, EM-G, and PC-C thank the editors for the invitation to participate in this special volume.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as potential conflicts of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Belaústegui, Z., Muñiz, F., Domènech, R., and Martinell, J. (2020). Ichnogeny and bivalve bioerosion: Examples from shell and wood substrates. Ichnos 27 (3), 277–283. doi:10.1080/10420940.2020.1744584
Belaústegui, Z., Muñiz, F., Mángano, M. G., Buatois, L. A., Domènech, R., Martinell, J., et al. (2016). Lepeichnus giberti igen. nov. isp. nov. From the upper Miocene of Lepe (Huelva, SW Spain): Evidence for its origin and development with proposal of a new concept, ichnogeny. Palaeogeogr. Palaeoclimatol. Palaeoecol. 452, 80–89. doi:10.1016/j.palaeo.2016.04.018
Bromley, R. G. (2004). “A stratigraphy of marine bioerosion,” in The application of ichnology to palaeoenvironmental and stratigraphic analysis. Editor D. McIlroy), 228, 455–479.
Bromley, R. G., and Asgaard, U. (1993). Endolithic community replacement on a Pliocene rocky coast. Ichnos 2, 93–116. doi:10.1080/10420949309380081
Bromley, R. G., and D’Alessandro, A. (1987). Bioerosion of the Plio-Pleistocene transgression of southern Italy. Riv. Ital. Paleontol. Stratigr. 93, 379–442.
Bromley, R. G., and Heinberg, C. (2006). Attachment strategies of organisms on hard substrates: A palaeontological view. Palaeogeogr. Palaeoclimatol. Palaeoecol. 232, 429–453. doi:10.1016/j.palaeo.2005.07.007
Bromley, R. G., and Martinell, J. (1991). Centrichnus, new ichnogenus for centrically pattemed attachment scars on skeletal substrates. Bull. Geol. Soc. Denmark 38, 243–252. doi:10.37570/bgsd-1990-38-21
Bromley, R. G., and Surlyk, F. (1973). Borings produced by brachiopod pedicles, fossil and Recent. Lethaia 6 (4), 349–365. doi:10.1111/j.1502-3931.1973.tb01203.x
Buatois, L., and Mángano, M. G. (2011). Ichnology: Organism-Substrate Interactions in Space and Time. Cambridge: Cambridge University Press, 358.
Castillo, C., Martín González, E., Yanes, Y., Ibáñez, M., Nuez, J., Alonso, M. R., et al. (2002). Estudio preliminar de los depósitos dunares de los Islotes del Norte de Lanzarote. Implicaciones paleoambientales. Geogaceta 32, 79–82.
Coello, J., Cantagrel, J. M., Hernán, F., Fúster, J. M., Ibarrola, E., Ancochea, E., et al. (1992). Evolution of the eastern volcanic ridge of the Canary Islands based on new K Ar data. J. Volcanol. Geotherm. Res. 53 (1–4), 251–274. doi:10.1016/0377-0273(92)90085-r
Dávid, Á., Szabolcs, B., and Fodor, R. (2008). Bioerosion on early-miocene oyster shells (Tardona Hills, Hungary). – Ichnia 2008 Cracow, Poland. Program Abstr. 45.
de Gibert, J. M., Domènech, R., and Martinell, J. (2004). An ethological framework for animal bioerosion trace fossils upon mineral substrates with proposal of a new class, fixichnia. Lethaia 37, 429–437. doi:10.1080/00241160410002144
de Gibert, J. M., Domènech, R., and Martinell, J. (2007). Bioerosion in shell beds from the Pliocene Roussillon Basin, France: Implications for the (macro)bioerosion ichnofacies model. Acta Palaeontol. Pol. 52, 783–798.
De la Nuez, J., Quesada, M. L., Alonso, J. J., Castillo, C., and Martín González, E. (1997b). “Edad de los Islotes en función de los datos paleontológicos,” in Los volcanes de los islotes del norte de Lanzarote (Madrid: Fundación César Manrique), 71–81.
De la Nuez, J., Quesada, M. L., and Alonso, J. J. (1997a). Los volcanes de los islotes del norte de Lanzarote. Madrid: Fundación César Manrique, 223.
Donovan, S. K., Jagt, J. W. M., and Lewis, D. N. (2011). Notes on some trace fossils and other parataxa from the Maastrichtian type area, southeast Netherlands and northeast Belgium. Neth. J. Geosciences 90, 99–109. doi:10.1017/s0016774600001050
Donovan, S. K. (2003). The ichnofossil Renichnus arcuatus mayoral, 1987 in the Pleistocene of Jamaica. Bull. Mizunami Foss. Mus. 30, 137–140.
El-Hedeny, M. M. (2007). Ichnology of the upper cretaceous (Cenomanian-Campanian) sequence of Western Sinai, Egypt. Egypt. J. Paleontology 7, 269–288.
Fürsich, F. T., Palmer, T. J., and Goodyear, K. L. (1994). Growth and disintegration of bivalve-dominated patch reefs in the Upper Jurassic of southern England. Palaeontology 37, 131–171.
Gutiérrez, M., Casillas, R., Fernández, C., Balogh, K., Ahijado, A., Castillo, C., et al. (2006). The submarine volcanic succession of the basal complex of Fuerteventura, Canary Islands: A model of submarine growth and emergence of tectonic volcanic islands. Geol. Soc. Am. Bull. 118 (7-8), 785–804. doi:10.1130/b25821.1
Hoșgör, I., and Okan, Y. (2010). Bioerosion structures on Crassostrea gryphoides (schlotheim, 1813) shells from the Salyan Formation (upper Burdigalian-lower Langhian), K. Maraș, southeastern Turkey. Türkiye Jeol. Bülteni 53, 45–62.
Jagt, J., van Rijsselt, W., and van Rijsselt, E. (2009). Opmerkelijke luiks-limburgse krijtfossielen: Deel 13. Honkvaste slakken. Natuurhistorisch Maandbl. 98 (8), 158–161.
Jagt, J. W. (2003). The ichnofossil genera Radulichnus and Renichnus in the Maastrichtian of The Netherlands and Belgium. Bulletin-Institut R. Sci. Nat. Belg. Sci. terre 73, 175–184.
Keen, M. (1961). A proposed reclassification of the gastropod family Vermetidae. Bull. Br. Mus., Nat. Hist. (Zool.) 7 (3), 183–213. pls. 54-55.
Martín-González, E., Castillo, C., Gutiérrez González, M., and Aguirre, J. (2001). Estudio paleoambiental de los depósitos litorales someros del Plioceno inferior de Fuerteventura (Islas Canarias). Rev. Española Paleont., Spec., 47–57. doi:10.7203/sjp.16.3.21615
Martín-González, E., Vera-Peláez, J. L., Castillo, C., and Lozano-Francisco, M. C. (2018). New fossil gastropod species (Mollusca: Gastropoda) from the upper Miocene of the Canary Islands (Spain). Zootaxa 4422, 191. doi:10.11646/zootaxa.4422.2.3
Martinell, J., and Domènech, R. (2009). Commensalism in the fossil record: Eunicid polychaete bioerosion on Pliocene solitary corals. Acta Palaeontol. Pol. 54, 143–154. doi:10.4202/app.2009.0115
Matamales-Andreu, R., Juárez, J., and Martinell, J. (2007). Estructures de macrobioerosió en Persististrombus latus (Gmelin, 1791) del Pleistocè superior de Mallorca (illes Balears, Mediterrània Occidental). Nemus 7, 19–29.
Mayoral, E. (1987). Acción bioerosiva del Mollusca (Gastropoda, Bivalvia) en el Plioceno inferior de la Cuenca del Bajo Guadalquivir. Rev. Española Paleont. 2, 49–58. doi:10.3989/egeol.88443-4548
Meco, J., Koppers, A. A., Miggins, D. P., Lomoschitz, A., and Betancort, J. F. (2015). The Canary record of the evolution of the North Atlantic Pliocene: New 40Ar/39Ar ages and some notable palaeontological evidence. Palaeogeogr. Palaeoclimatol. Palaeoecol. 435, 53–69. doi:10.1016/j.palaeo.2015.05.027
Ortiz, J. E., Torres, T., Yanes, Y., Castillo, C., Nuez, J. D. L., Ibáñez, M., et al. (2006). Climatic cycles inferred from the aminostratigraphy and aminochronology of Quaternary dunes and palaeosols from the eastern islands of the Canary Archipelago. J. Quat. Sci. 21 (3), 287–306. doi:10.1002/jqs.962
Pickerill, R. K., and Narbonne, G. M. (1995). Composite and compound ichnotaxa: A case example from the Ordovician of Québec, eastern Canada. Ichnos 4, 53–69. doi:10.1080/10420949509380114
Pickerill, R. K. (1994). “Nomenclature and taxonomy of invertebrate trace fossils,” in The Palaeobiology of trace fossils. Editor S. K. Donovan (Chichester, UK: John Wiley & Sons), 3–42.
Radwański, A. (1969). Lower Tortonian transgression onto the southern slopes of the Holy Cross Mountains. Acta Geol. Pol. 19, 1–64.
Radwański, A. (1977). “Present-day types of trace in the Neogene sequence; their problems of nomenclature and preservation,” in Trace fossils 2. Editors T. P. Crimes, and J. C. Harper (Liverpool: Geological Journal Special Issue), 9, 227–264.
Rosso, A. (2008). Leptichnus tortus ichnosp. nov., a new cheilostome etching and comments on other bryozoan-produced trace fossils. Studi Trentini Sci. Nat. Acta Geol. 83, 75–85.
Santos, A., and Mayoral, E. (2006). Bioerosive structures of sclerozoan foraminifera from the lower Pliocene of southern Spain: A contribution to the palaeoecology of marine hard substrate communities. Palaeontology 49, 719–732. doi:10.1111/j.1475-4983.2006.00560.x
Savazzi, E. (1996). Adaptation of vermetid and siliquariid gastropods. Palaeontology 39 (1), 157–177.
Savazzi, E. (1999a). “Cemented and embedded gastropods,” in Functional morphology of the invertebrate skeletonJ. Wileyeditor Chichester, 183–195.
Taddei Ruggiero, E. (1999). Bioerosion on brachiopods shells – A cenozoic perspective. Earth Environ. Sci. Trans. R. Soc. Edinb. 45, 169–172.
Taddei Ruggiero, E., and Bitner, M. A. (2008). Bioerosion on brachiopods shells – A cenozoic perspective. Earth Environ. Sci. Trans. R. Soc. Edinb. 98, 369–378.
Taylor, P. D., Wilson, M. A., and Bromley, R. G. (2013). Finichnus, a new name for the ichnogenus Leptichnus taylor, Wilson and Bromley, 1999, preoccupied by Leptichnus simroth, 1896 (Mollusca, gastropoda). Palaeontology 56 (2), 456. doi:10.1111/pala.12000
Uchman, A., Kleeman, K., and Rattazzi, B. (2017). Macroborings, their tracemakers and nestlers in clasts of a fan delta: The Savignone Conglomerate (lower Oligocene), Northern Apennines, Italy. Neues Jahrb. Geol. Paläontol., Abh. 283, 35–51. doi:10.1127/njgpa/2017/0625
Uchman, A., Stachacz, M., and Salamon, K. (2018). Spirolites radwanskii n. igen. n. isp.: Vermetid gastropod attachment etching trace from the middle Miocene rocky coast of the Paratethys, Poland. J. Paleontol. 92, 883–895. doi:10.1017/jpa.2017.95
Wisshak, M., Knaust, D., and Bertling, M. (2019). Bioerosion ichnotaxa: Review and annotated list. Facies 65 (24), 24. doi:10.1007/s10347-019-0561-8
Wisshak, M. (2017). Taming an ichnotaxonomical pandora’s box: Revision of dendritic and rosetted microborings (ichnofamily: Dendrinidae). Eur. J. Taxon. 390, 1–99. doi:10.5852/ejt.2017.390
Wisshak, M., Tribollet, A., Golubic, S., Jakobsen, J., and Freiwald, A. (2011). Temperate bioerosion: Ichnodiversity and biodiversity from intertidal to bathyal depths (Azores). Geobiology 9, 492–520. doi:10.1111/j.1472-4669.2011.00299.x
Keywords: bioerosion structures, vermetid etching trace fossil, fixichnia, compound trace fossil, Canary Islands, Miocene, Pliocene, ichnogenus Renichnus
Citation: Verde M, Castillo C, Martín-González E, Cruzado-Caballero P, Mayoral E and Santos A (2022) A new miocene–pliocene ichnotaxon for vermetid anchoring bioerosion structures. Front. Earth Sci. 10:906493. doi: 10.3389/feart.2022.906493
Received: 28 March 2022; Accepted: 29 June 2022;
Published: 22 August 2022.
Edited by:
Sally Walker, University of Georgia, United StatesReviewed by:
Olev Vinn, University of Tartu, EstoniaMao Luo, Nanjing Institute of Geology and Paleontology (CAS), China
Copyright © 2022 Verde, Castillo, Martín-González, Cruzado-Caballero, Mayoral and Santos. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mariano Verde, verde@fcien.edu.uy
†ORCID: Eduardo Mayoral, orcid.org/0000-0002-2616-8640; Carolina Castillo, orcid.org/0000-0003-3381-7490; Esther Martín-González, orcid.org/0000-0001-5659-2197; Mariano Verde, orcid.org/0000-0002-0735-1124; Penélope Cruzado-Caballero, orcid.org/0000-0002-5819-8254; Ana Santos, orcid.org/0000-0001-6136-8376
 Mariano Verde
Mariano Verde Carolina Castillo
Carolina Castillo Esther Martín-González
Esther Martín-González Penélope Cruzado-Caballero2,4,5,
Penélope Cruzado-Caballero2,4,5,  Eduardo Mayoral
Eduardo Mayoral Ana Santos
Ana Santos